Clinical Trial Evaluating the Safety and Efficacy of Pepinemab in Combination with Pembrolizumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck
A clinical trial is a controlled study to evaluate the safety and efficacy (how well it works) of an experimental drug treatment. These studies are required before a drug can be approved by a regulatory agency such as the Food and Drug Administration (FDA) or European Medicines Agency (EMA). Patients interested in participating in a clinical trial must meet certain eligibility criteria and be willing to follow directions of the physician in charge of the study.
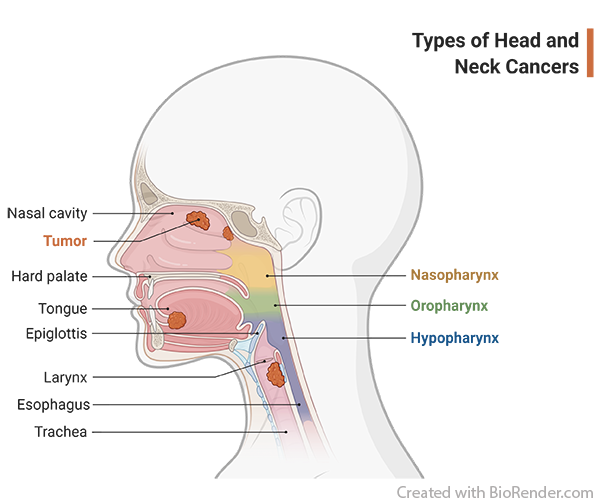
What is head and neck cancer?
Head and neck cancers usually begin in the squamous cells that line the mucosal surfaces of the head and neck (for example, those inside the mouth, throat, and voice box). Symptoms may include a lump or a sore in the mouth or the neck that does not heal and may be painful, a sore throat that does not go away, difficulty in swallowing, and a change or hoarseness in the voice. It is important to check with your doctor if you experience any of these symptoms. Risk factors such as family history, HPV infection and lifestyle, can lead to mutations in the squamous cells which may progress to cause cancer. Head and neck cancer may continue to grow and spread locally and/or to the lymph nodes in the neck. When this happens, the cancer is called metastatic squamous cell carcinoma.
There is currently no cure for head and neck cancer, but current treatment options include surgery, radiation therapy, chemotherapy, targeted therapy and immunotherapy.
Trial Purpose
The purpose of the study is to evaluate the safety and tolerability of pepinemab in combination with pembrolizumab in patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). The primary hypothesis being tested is whether the combination of pepinemab + pembrolizumab will be safe and provide clinical benefit in patients with R/M HNSCC.
Active, not recruiting
View Trial on ClinicalTrials.gov
National Trial Reference Number
NCT04815720
Trial Details
About the Trial
All patients who enroll in the trial will receive standard of care pembrolizumab, and will also receive pepinemab on the same day.
Phase
Phase 1b/Phase 2
Dates
Actual Study Start Date
August 9, 2021
Estimated Primary Completion Date
September 4, 2023
Estimated Study Completion Date
September 4, 2023
Sponsor
Vaccinex, Inc.
Collaborators
Merck Sharp & Dohme Corp.
Products
pepinemab
pembrolizumab
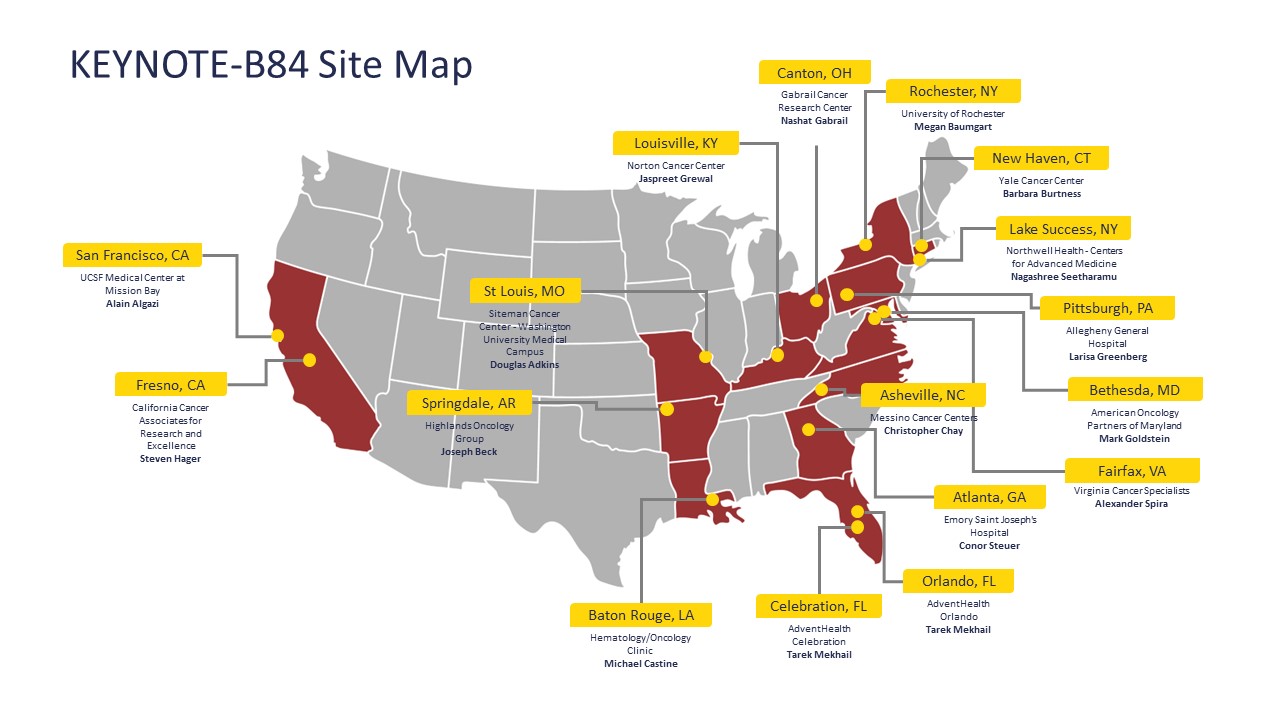
Trial Locations